Rank the Following Bonds From Most Polar to Least Polar
Show transcribed image text Expert Answer. 100 6 ratings take the electronegativity difference of both atoms in all.

Solved Rank The Following Bonds From Highest Polarity To The Lowest 1 Most Polar 4 Least Polar N Ci Select C Si Select O P Select A C Select
Previous question Next question.

. Experts are tested by Chegg as specialists in their subject area. As others have written the C-C bond is the least polar as the electronegativity difference between them is 0. Where would a series of non-polar amino acids most likely be located in a protein that is found in the cytosol of an.
CCl CI CBr c. Rank the following bonds from highest polarity to the lowest. Which of the following bonds would be the most polar without being considered ionic.
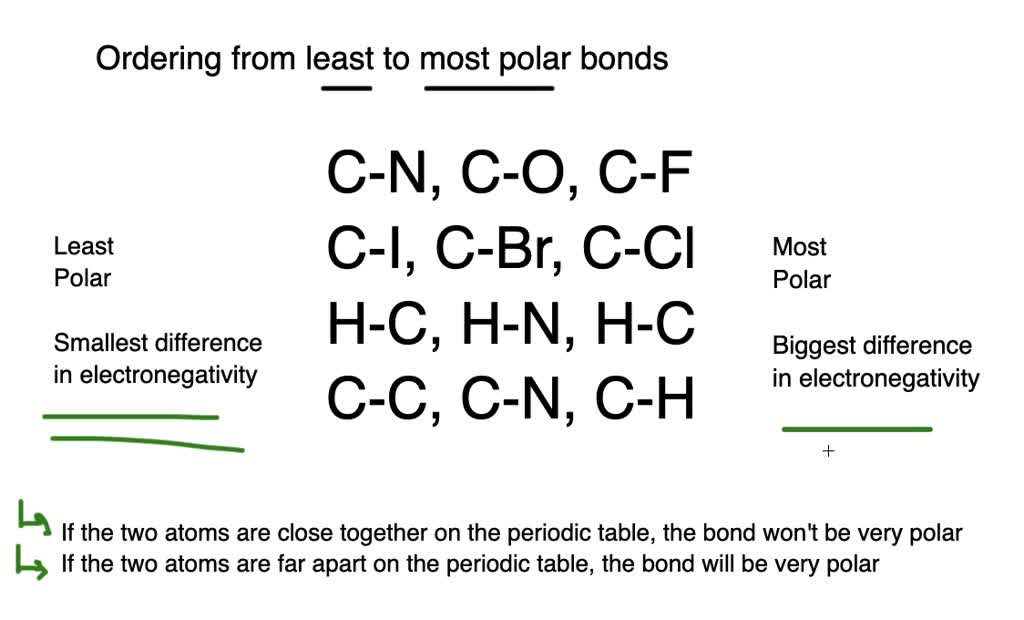
For the rest of the rank order it would be C-N C-Cl C-O and C-F with C-F being the most polar. Increasing the electronegativity difference lead to a more polar bond. The polarity of a bond depends on electronegativity difference between the two atoms sharing the bonding electrons.
Weve got the study and writing resources you need for your assignments. C- O N- Cl Least polar Most polar. CH2F2 The most symmetrical molecule CBr4 would be the least polar.
1 most polar4 least polar NCI Select C-Si SeleLets keep an eye on the content below. Use numbers from 1 least to 4 most C-F. Is SeF4 polar or nonpolar.
Elect to negativity polarity difference electronegaitivity C 255 228 344 lectronegativity difference between 0 zero c-c C-H 035 C-o 089 - 124 o-H correct order of polarity a C-C Cl c0 most polar least. C-C By signing up youll get thousands of. Rank the following bonds in the order of increasing polarity least polar to most polar least polar C-C 0-H C-O C-H most polar least polar C-C C-H C-O 0-H most polar least polar C-C C-H 0-H C-O most polar least polar C-H C-C C-O 0-H most polar Determine the bond.
Who are the experts. Start your trial now. Click here to get an answer to your question Rank the bonds from most polar to least polar.
Rank the bonds from most polar to least polar. 1 being the least polar and 4 being the most polar. Rank the following bonds from least polar to most polar.
We review their content. Most polar C-F Second most polar C-O Least polar C-N Assign formal charges to the atoms in the Lewis structure below. A C-O C-F C-N b C-CI C-I C-Br c H-O H-N H-C d C-H C KittyGirl8265 KittyGirl8265 09072020 Chemistry College answered Rank the bonds from most polar to least polar.
When electros are shared equally between the bonded atoms or when the polar bonds in a bigger molecule cancels out each other then a a molucule is non polar. Start your trial now. First week only 499.
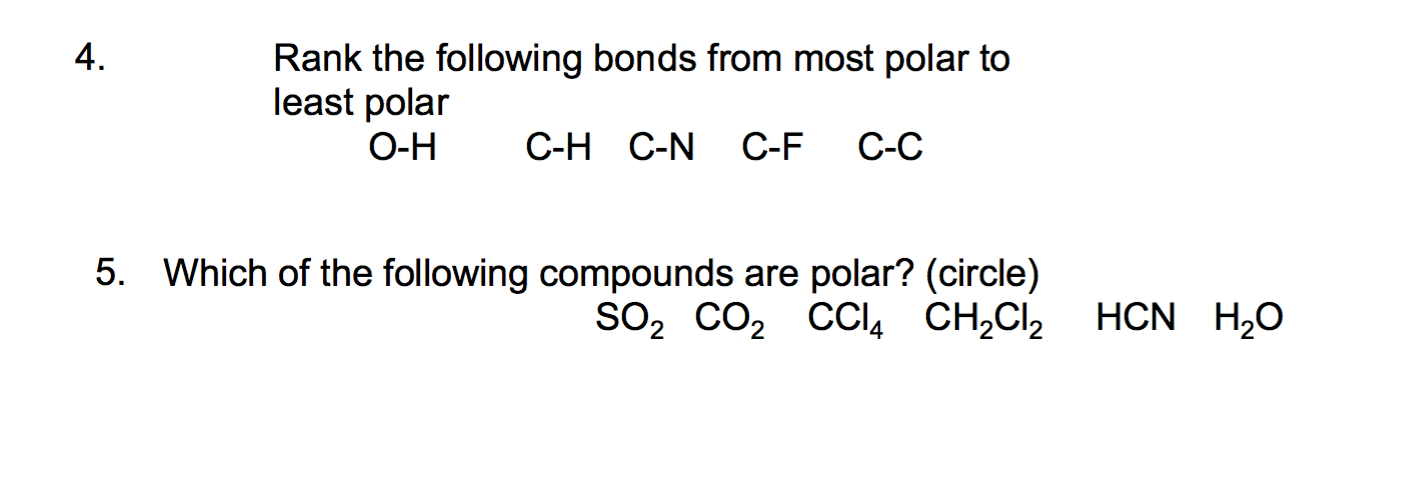
Solution for Rank the following bonds from least polar to most polar. Circle SO_2 CO_2 CCL_4 CH_2CL_2 HCN H_2O. Given the electronegativity of each of the following atoms rank the bonds from most polar number 1 to least polar number 5.
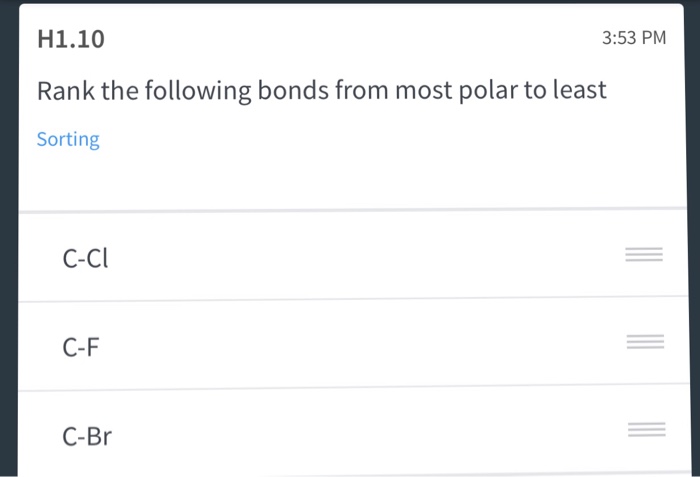
Rank the following solutions from least polar to most polar. Match each bond to its appropriate classification. Rank the following bonds from most polar to least polar O-H C-H C-N C-F C-C Which of the following compounds are polar.
Si-Cl P-Cl Mg-Cl S-Cl. Thus and according to Linus Pauling scale of the electronegativity rightarrow. Rank the following bonds from most polar to least Sorting C-Cl C-F C-Br.
Chemistry questions and answers. Rank the following bonds from least polar to most polar. O 35 N 30 C 25 H 21 1.
Given the electronegativity of each of the following atoms rank the bonds from most polar number 1 to least polar number 5. See the answer See the answer See the answer done loading. A 50 isopropanolH2O--- 2.
Rank on a scale of 1-4. Solution for Rank the following bonds in the order of increasing polarity least polar to most polar a least polar C-C. Rank the following bonds from least polar to most polar.
Mg-O C-O O-O Si-O N-O. Rank the following from smallest to largest atomic radius. Li-C Polar Covalent H-C Non-polar Covalent O-C Polar Covalent N-C Non-polar Covalent Rank the following bonds from most polar to least polar.
Chemistry questions and answers. Chemistry questions and answers. Which of the following bonds would be the least polar yet still be considered polar covalent.
Rank the following bonds from least polar to most polar C- C-N N Cl Least polar Most polar. This is the best answer based on feedback and ratings. 0 35 N 30 C 25 H 21.
Matter is composed of. View the full answer. Rank the following bonds from most polar to least polar.
HO HN HC d. 1 most polar4 least polar NCI Select C-Si Sele The following solution is suggested to handle the subject Rank the following bonds from highest polarity to the lowest. C-C Chlorine atomic number 17 Has 7 valance electrons Most likely to gain an electron.
First week only 499. CO CF CN b. Rank the following bonds from least polar to most polar.
Basically I would say that you have to look at the difference of electronegativity of the atoms on opposite sides of the molecules. This problem has been solved. Aus in polar bonds elect more electronegative atom pull election density towards itsey This polarity depends on electronegativity difference between two atoms.
Solved Rank The Following Bonds From Most Polar To Least Chegg Com
Solved Rank The Following Bonds From Most Polar To Least Chegg Com
Solved Rank The Bonds From Most Polar To Least Polar A Quad Mathrm C Mathrm O Mathrm C Mathrm F Mathrm C Mathrm N B Quad Mathrm C Mathrm Cl Mathrm C Mathrm I Mathrm C Mathrm Br C Quad Mathrm H Mathrm O Mathrm H
Comments
Post a Comment